Digest Prep - First Bead Clean for Enzyme Testing
Bead Clean for Enzyme Testing
Samples selected for test: 401, 419, 759
Date Performed: January 26th, 2021
These samples were chosen based on location, date collected, sex, and DNA concentration/gel quality
- Set up strip tube for each sample (8 total reactions per sample)
-
Added volume necessary for 500ng DNA (this number was calculated in this spreadsheet:https://docs.google.com/spreadsheets/d/1njUXXOcOWUZv8l4SMtWlhzyxg0ekKZ7k4dtOU3trQ-I/edit#gid=0)
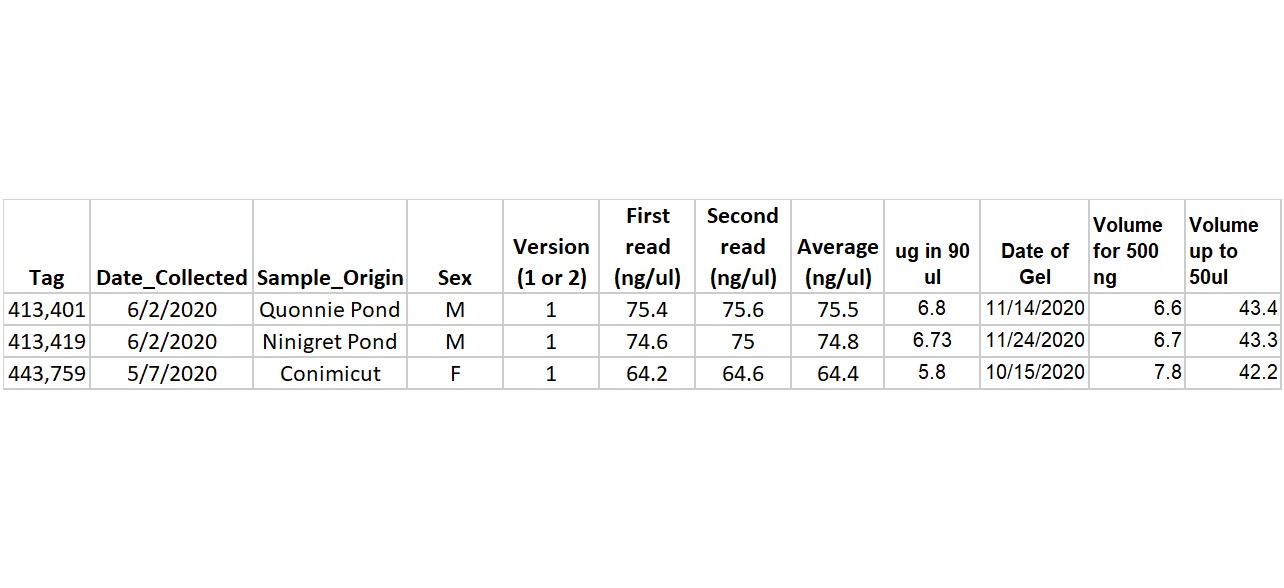
- Added remainder volume up to 50ul with Nuclease Free Water
- Added 50ul Kappa beads to each sample and pipetted up and down to mix (10x)
- Placed on orbital mixer in magnet plate for 15 minutes on 200
- Made 80% ethanol mix (5omL conical 40mL 100% ethanol and 10mL nuclease free water)
- Took samples off mixer
- Removed clear liquid from each tube and expelled into waste trough
- Set pipette to ~1ul(this may be off) to carefully suck up any remaining liquid
- Added 200ul 80% ethanol to each tube on side without bead
- Removed 200ul 80% ethanol without disturbing bead
- Added 200ul 80% ethanol to each tube on side without bead
- Removed 200ul 80% ethanol without disturbing bead
- Used a p20 to remove any excess ethanol in each tube
- Waited few minutes for any ethanol to evaporate
- Took tubes off magnet plate
- Resuspended beads in 70ul nuclease free water
- Put tubes back on shaker
- Labelled new tubes for cleaned DNA
- Put tubes back on magnet until liquid clears
- Removed clear liquid from each tube and put in corresponding new tube
- Stored in fridge for next step
Written on January 27, 2021