Digestion (Testing) with Plate B
1X Bead Clean, Qubit, Digestion
Dates Performed: September 2nd, 9th, 14th
THIS FIRST SECTION IS JUST FOR THE FIRST ROW (A) OF PLATE B 1X Bead Clean
- Thawed Plate B, vortexed, centrifuged
- Set multi channel pipette to 51ul to remove Row A of plate B into set of strip tubes (10 samples)
- Made fresh conical of 80% ethanol
- Warmed 1000ul Tris HCL to 37C
- Added 50ul beads to each sample with multichannel and mixed by pipetting
- Put samples on orbital shaker for 15 minutes at 200RPM
- Set up strip tubes for each waste removal (3 total)
- Placed samples on magnet on shaker for 3 minutes
- Once samples were clear, removed 90ul clear fluid and ejected into waste strip tube 1
- Added 200ul 80% ethanol to each sample tube
- Removed 200 ul ethanol from each sample tube and kept is strip tube 2 for waste
- Added 200ul 80% ethanol to each sample tube
- Removed 200 ul ethanol from each sample tube and kept is strip tube 3 for waste
- Went back in with p20 set to 20 to remove any residual ethanol.
- Let first 5 samples dry with caps off then added 70ul warmed Tris HCL to each sample
- Opened 5 remaining tubes to dry then added 70ul warmed Tris HCL to each sample
- Placed tubes back on shaker for five minutes
- Placed tubes on magnet on shaker for two minutes until clear
- Removed clear liquid and put in new set of strip tubes
Only made enough Qubit master mix for 8 samples
| Sample | Run 1 |
|---|---|
| S1 | 191.21 |
| S2 | 22154.07 |
| 1 | 6.48, 6.76 |
| 2 | 6.60, 7.07 |
| 3 | 5.70, 5.90 |
| 4 | 5.88, 6.16 |
| 5 | 4.50, 4.70 |
| 6 | 2.52, 2.62 |
| 7 | 7.02, 5.76 |
| 8 | 5.62, 6.24 |
Made digestion master mix (enough for 12 samples) but still ended up needing to make more. I am unsure where this error is coming from, there should have been 20ul extra. Started digest in thermocyler 4 at 3:30
Post digest performed 1.5X bead clean
- Removed strip tube from freezer to thaw and spin down
- Made fresh conical of 80% ethanol
- Added 120ul beads to each sample with multichannel and mixed by pipetting
- Put samples on orbital shaker for 15 minutes at 200RPM
- Placed samples on magnet on shaker for 3 minutes
- Once samples were clear, removed 190ul clear fluid and ejected into waste
- Added 200ul 80% ethanol to each sample tube
- Removed 200 ul ethanol from each sample tube
- Added 200ul 80% ethanol to each sample tube
- Removed 200 ul ethanol from each sample tube
- Went back in with p20 set to 20 to remove any residual ethanol.
- Let samples dry
- Added 33ul Tris HCL to each sample
- Placed tubes back on shaker for five minutes
- Placed tubes on magnet on shaker for two minutes until clear
- Removed clear liquid and put in new set of strip tubes
Post 1.5X Clean Qubit Reads
| Sample | Run 1 |
|---|---|
| S1 | 181.22 |
| S2 | 22197.32 |
| 1 | 9.20 |
| 2 | 8.90 |
| 3 | 7.34 |
| 4 | 7.92 |
| 5 | 6.08 |
| 6 | 3.40 |
| 7 | 7.26 |
| 8 | 7.22 |
Plate Reader Results from Row A
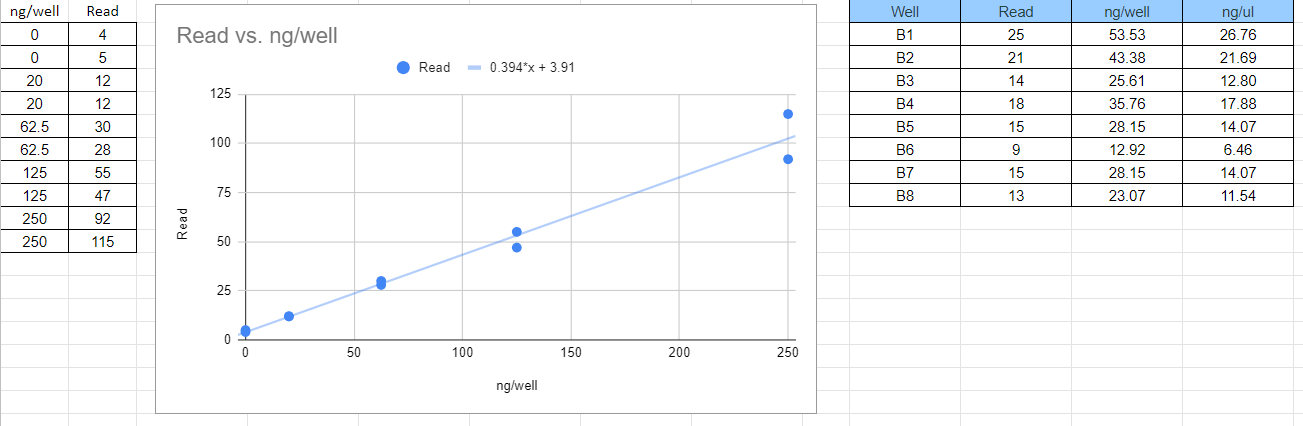
FOLLOWING SECTION FOR REMAINDER OF PLATE B
Followed 1X bead clean protocol. Did not warm Tris HCL and did not leave beads in. This plate had 7 almost full rows so I split the clean in half and did the entire clean on the top three rows then the entire clean on the bottom 4 rows. I think this should help with not having the beads dry too quickly. After adding 70ul Tris HCL I put the plate on the orbital shaker for ten minutes then on the magnet on the orbital shaker until the liquid was clear. Then I moved the clear liquid to a new plate for digestion. Since I have been running out of digestion master mix I made enough for 100 samples.
| Number of columns | Volume to Tube |
|---|---|
| 10 | 130 |
| 9 | 130 |
| 8 | 130 |
| 10 | 130 |
| 10 | 130 |
| 9 | 130 |
| 8 | 130 |