Re-run Pool 1 Blue Pippin, Pools 3,4,5 PCR and Index Addition
Run Pool 1 Size Selection with Tight Setting, PCR 3,4,5
Date Performed: January 26th, 2022
PCR and Index Addition USE FILTER TIPS FOR ALL PIPETTING
- For each pool you are going to set up 6 PCR reactions. This minimizes possible PCR biases but also amplifies the libraries to a good amount. If you are amplifying more than one pool at a time (say 5), it can be easier to carry out all the reactions on a 96 well plate instead of individual strip tubes
- Set up a plate or however many tubes you’ll need to have 6 wells/tubes per pool. Label A, B, C etc as these are very temporary
- Thaw size-selected samples, ready mix, and 50X, 70X primers on ice. Vortex and spin down all before using
- Each pool needs a master mix because each pool gets their own 50X-70X primer pair
- Make a master mix for each pool (ex for 5 pools, you would make 5 mixes, each with their specific primer pair):
- 10µl of 2X KAPA HiFi Hot Start Ready Mix * 6.1 = 66ul
- 4µl water * 6.1 = 26.4ul
- .5μl 20uM PCR primer 50X *6.1 = 3.3ul
- .5μl 20uM PCR primer 70X *6.1 = 3.3ul
- Vortex and spin down the master mixes
- Pipette 15ul of each master mix into their planned 6 tubes or wells
- Add 5ul of DNA from a size-selected pool to their respective wells, and repeat for the other pools
- Cover the plate with a foil seal, vortex to mix, and spin down
- Put in the Thermocycler for amplification, 12 cycle RAD PCR program under JONP login
- Samples can be frozen afterwards, or continue to clean-up
Notes on PCR
Pool 3 Index Pair = 506/706 Pool 4 Index Pair = 507/707 Pool 5 Index Pair = 508/708
Blue Pippin Pool 1
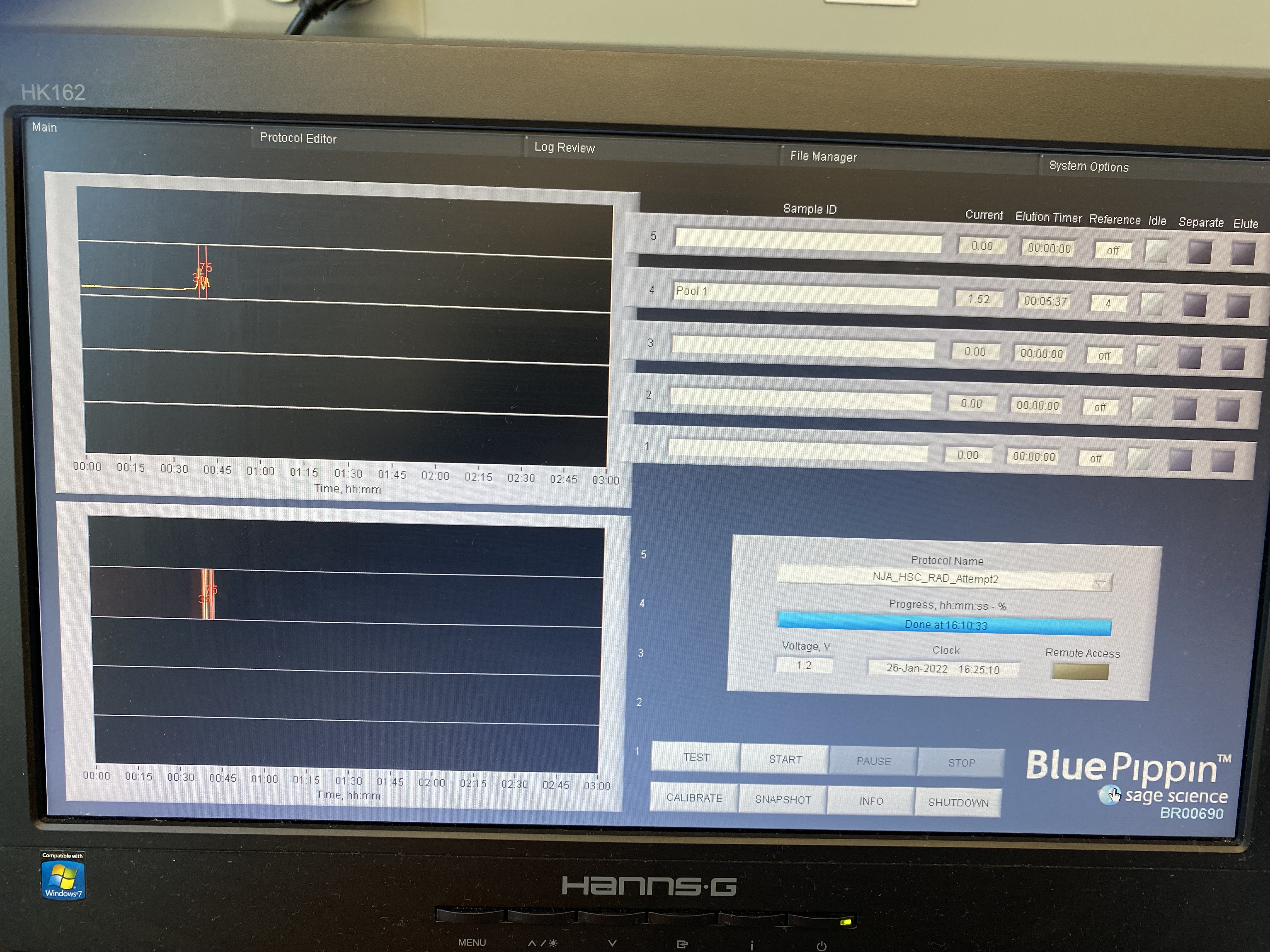
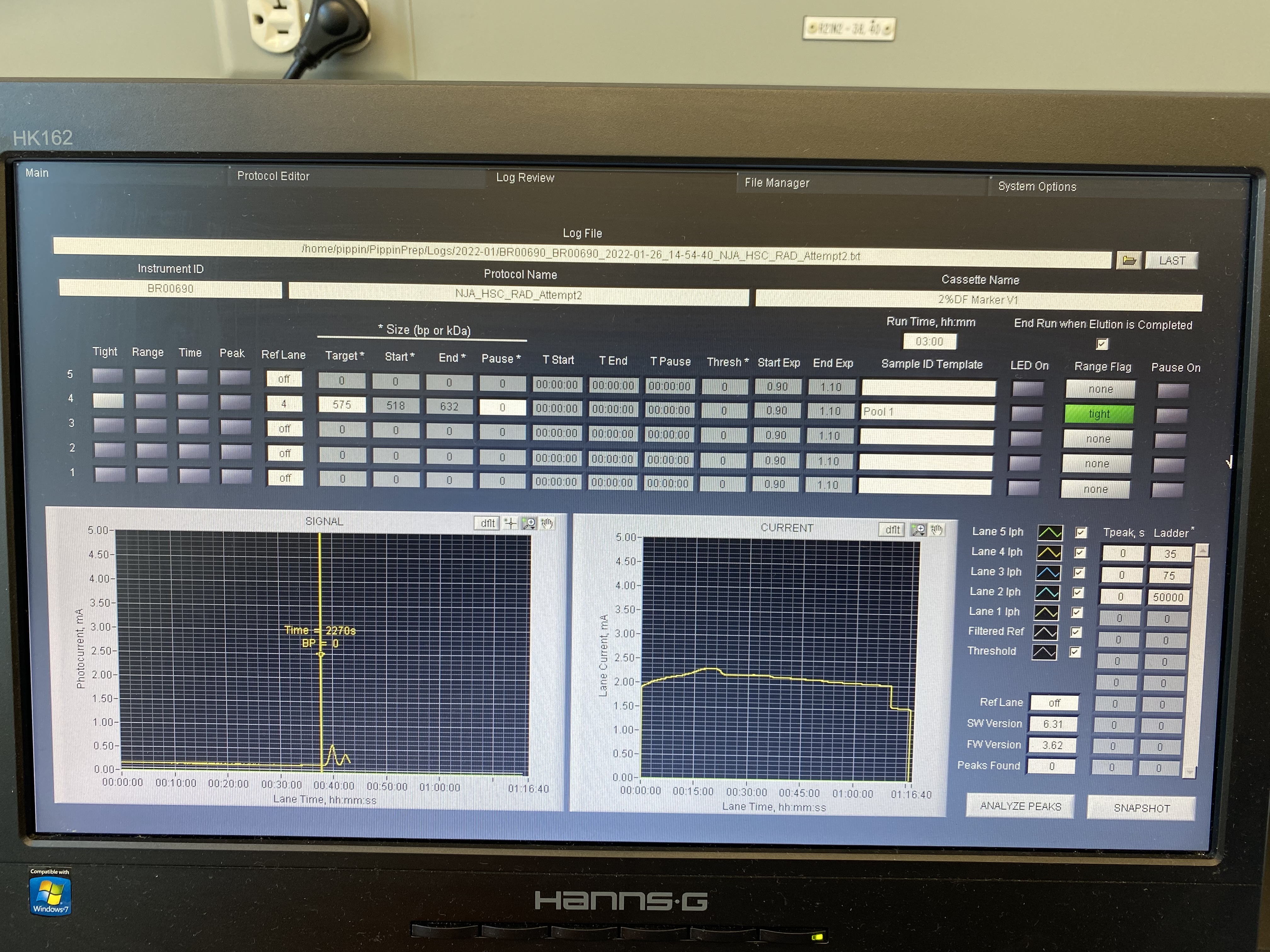
Written on January 26, 2022