Second Attempt Adapter Ligation Dilutions
Diluted Plates for Adapter Ligation and Qubit Testing
Dates Performed: July 27th and 29th and August 3rd
July 27th
Thawed plates A-E that contained extra DNA from cleaned and digested samples in step 10.
Selected 2 samples from each plate to quantify on Qubit
| Sample | Run 1 | Run 2 |
|---|---|---|
| S1 | 148.17 | |
| S2 | 15810.98 | |
| A - 754 | 8.70 | 8.04 |
| A - 355 | 20.4 | 18.1 |
| B - 605 | 16.3 | 15.8 |
| B - 759 | 9.48 | 9.16 |
| C - 787 | 14.9 | 13.8 |
| C - 485 | 14.2 | 13.8 |
| D - 440 | 19.9 | 20.4 |
| D - 752 | 14.6 | 14.3 |
| E - 431 | 14.7 | 14.9 |
| E - 788 | 13.9 | 13.6 |
Given the above results I am confident my samples post digest have adequate DNA.
Plate volume calculations:
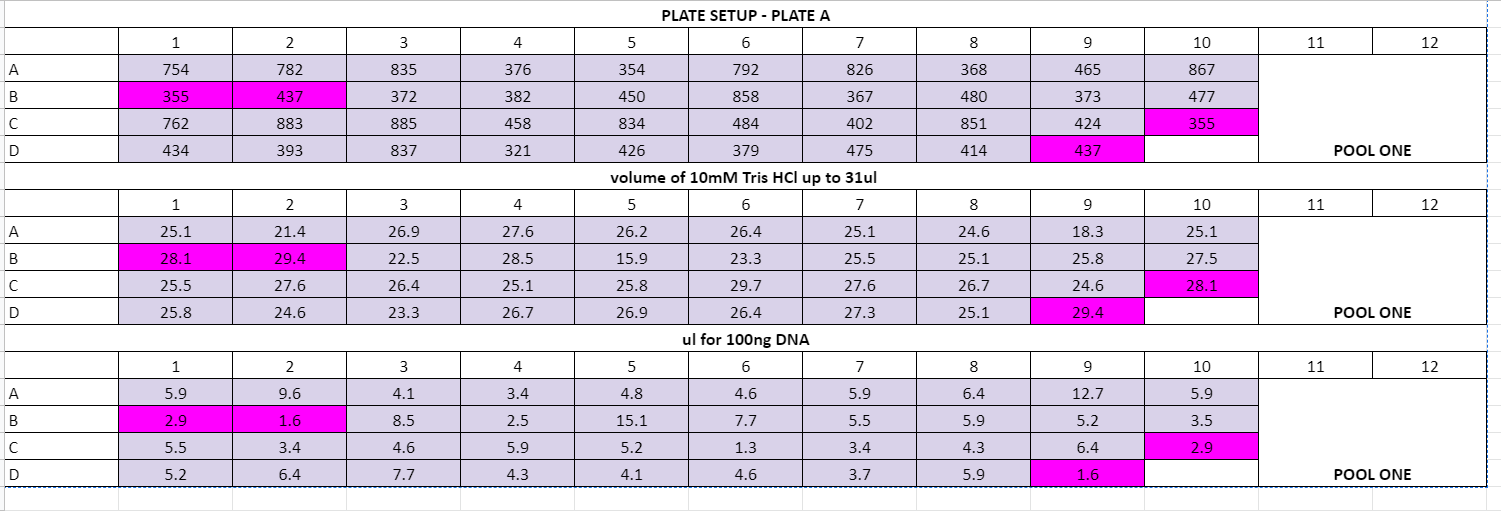
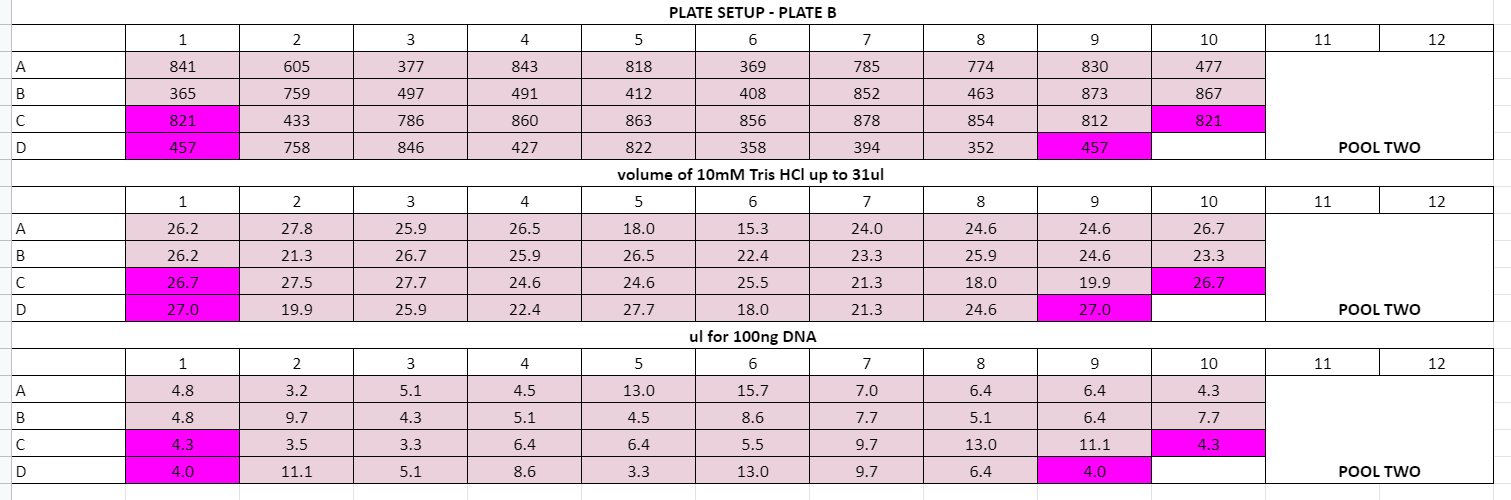
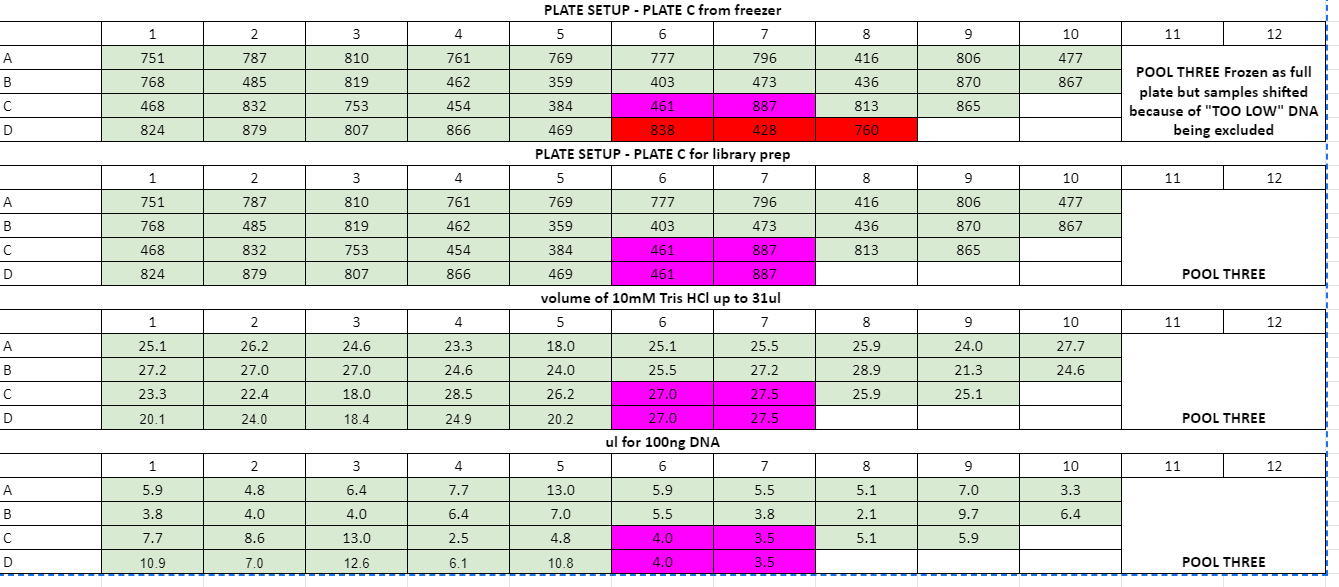
July 29th
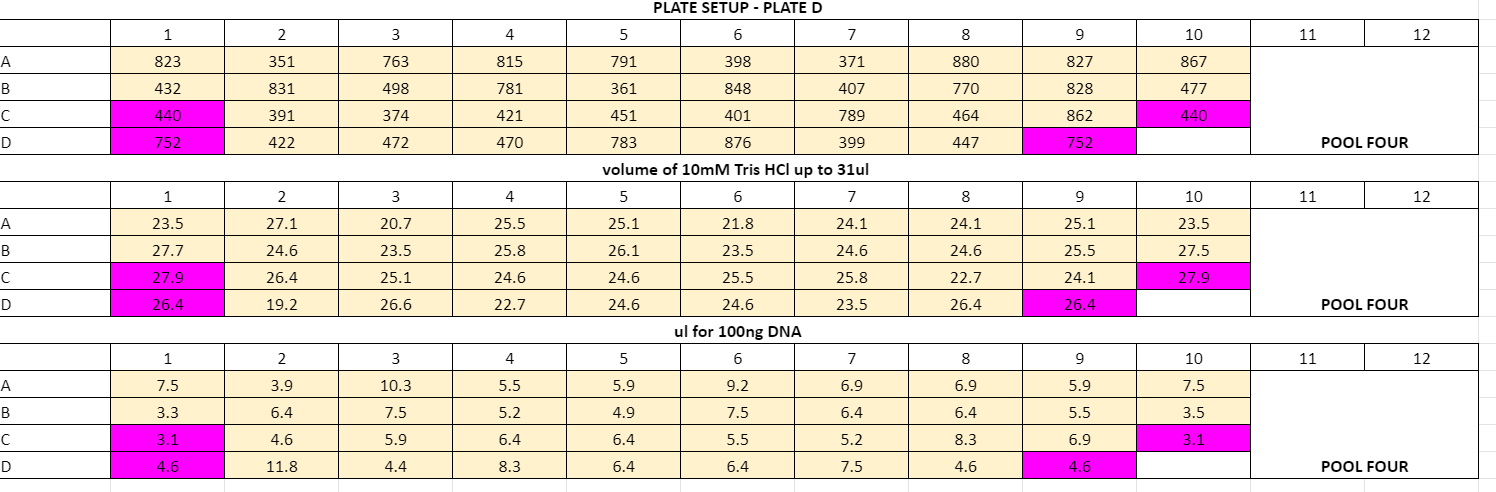
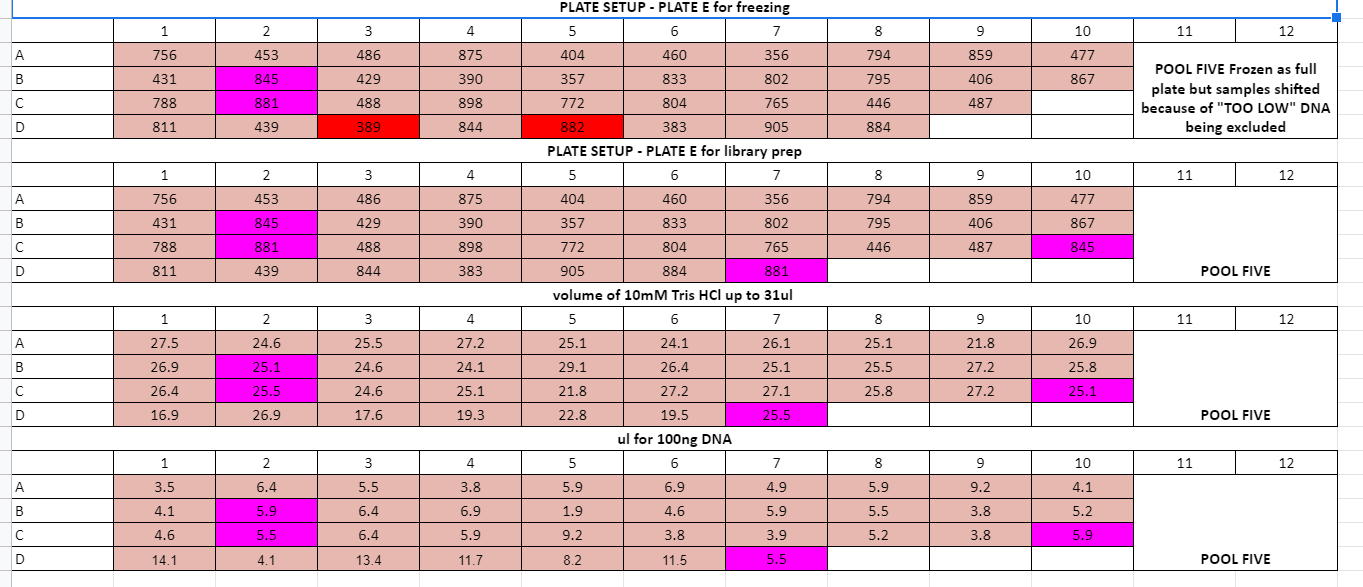
Ran adapter ligations for plates B, C, D, E
LMM for 2 plates
328ul 10X Ligation buffer
82ul T4 Ligase
Made LMM for plates D and E together and C and B together
See full adapter ligation steps below, because frozen left over DNA this process did not have beads in (step 10)
Adapter Ligation USE FILTER TIPS FOR ALL PIPETTING
Make the Ligation Master Mix (LMM) on ice. Vortex and spin down the buffer before use. Flick to mix the ligase and spin down. Do not vortex the ligase
4μl of 10X ligation buffer * number of samples+0.5 =
1μl of T4 ligase * number of samples+0.5 =
Flick to mix the LMM and spin down, keep on ice
Aliquot the LMM into strip tubes for multichannel pipetting into each well. Each well gets 5ul of LMM. Use rows or columns, whichever is easier to pipette. Keep on ice (Purple trays if available)
Aliquot out the adapters into strip tubes for easy addition to your samples (by going down the column and across the rows). Make one set of strip tubes for i5 adapters (added down columns) and make one set of strip tubes for i7 adapters (added across rows). Each well will get 2ul of the correct i5 adapter and 2ul of the correct i7 adapter. Keep on ice
Add 5ul of LMM to each well in the plate using the multichannel
Add 2ul of the correct i5 indexes by multichannel pipetting down columns
Add 2ul of the correct i7 indexes by multichannel pipetting across rows
Make sure that the ligation test sample(s) get LMM and adapters, however it does not matter which adapters they get
Pipette mix the wells with a p200 pipette set to 25ul
Foil seal the plate and spin it down in the large centrifuge
Put the plate in the Thermocyclers in the RAD LIGA program in the JONP login (password 1234). This takes between 4-5 hours to run. The program goes to a 4 degree hold afterwards
The plate can be taken out and frozen (because no bead in) until pooling\
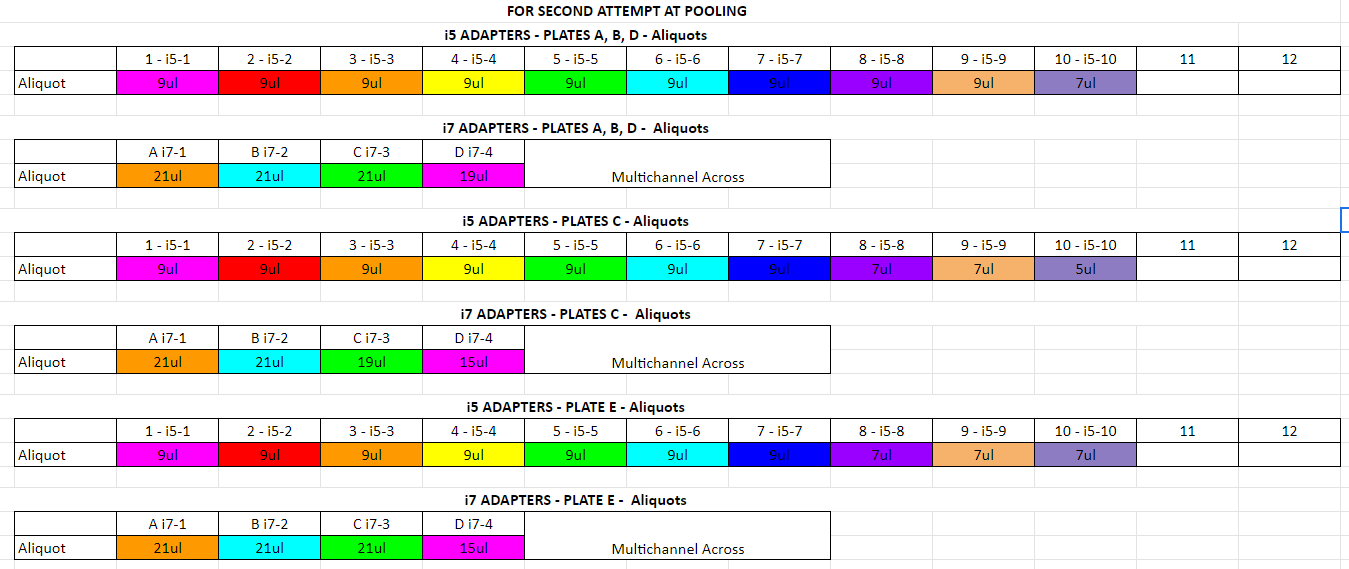
- add 1ul per tube for error for adapters
August 3rd Ran adapter ligation for plate A Needed for i7 adapters so I diluted i71-4 as 2.08ul annealed stock and 197.92ul 1x annealing buffer